This topic covers elements, mixtures and compounds.
Reflections: This topic was rather much a HUGE headache for me. First of all, as I had previously said, I am a TERRIBLE memorizer ( pardon the English). There were 20 elements to memorize and i also had to remember the groups and periods. Well, I did it eventually but that was not even close to the hard part. The hard part was the mixtures and compounds. I had to differentiate between a compound and a mixture, and also to remember which mixtures were mixed from what and which compounds were formed from which elements. This is kind of hard, considering the fact that there are 16 million organic (carbon-containing) compounds in the world. And those are only the carbon containing ones. Also, as I did research on this topic, unexpectedly I learnt something very interesting. Somehow, I saw the wiki page on elements had the link 'Matter', so I clicked on the link and read the page. I learnt that not everything in the world is made up of matter. In fact, less than only 17%of matter in the universe is made up of ordinary matter. The rest are a mysterious kind of matter called dark matter which naturally accounts for 83%.
Wednesday, September 14, 2011
Sunday, September 11, 2011
Acknowledgements
Information used in this blog are from various websites. The credits go to Wikipedia, Google Images, YouTube and the powerpoint slides in the Secondary 1 science wiki.
Saturday, September 10, 2011
Personal Work Samples
Although I did not participate in any science sabbaticals, but I read magazines like National Geographic and also I have many encyclopedias to read. The National Geographic is for updating me on current science issues and new discoveries, and the encyclopedias are for increasing my knowledge. Here are some photos! Enjoy!
Growth in Science
In my primary school, I was taught a lot on theory but never actually had the opportunity to try out all those experiments. Now, I am taught a lot more and i can also do practical instead of just learning about the experiment in theory. I feel that I learn things better when I actually work on them, as I can remember things more when I experience them instead of memorizing theories. This helps a lot when it comes to memorizing things for me. Also, secondary school science has shown me science in a new light. I used to think science was boring, but, after doing practical, they became a lot more interesting to me.
Termly Performance
For my Term 1 test, I scored 68 marks.
For my Term 2 test, I scored 60 marks.
For my Term 3 test, I scored 65 marks.
I will aim for an A in the EOY exams.
For my Term 2 test, I scored 60 marks.
For my Term 3 test, I scored 65 marks.
I will aim for an A in the EOY exams.
Cells, Tissues, Organs and Systems
Cells are the basic unit of our body. Cells group together to form tissues. Many tissues in turn form organs. Each system consists of organs. So, our body is built on this basis. Cells > Tissues > Organs > Systems.
In this chapter we learnt about the different type of cells, tissues and organs.
The cell is the functional basic unit of life. It was discovered by Robert Hooke and is the functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. Some organisms, such as most bacteria, are unicellular (consist of a single cell). Other organisms, such as humans, are multicellular. Humans have about 100 trillion or 1014 cells; a typical cell size is 10 µm and a typical cell mass is 1 nanogram. The longest human cells are about 135 µm in the anterior horn in the spinal cord while granule cells in the cerebellum, the smallest, can be some 4 µm and the longest cell can reach from the toe to the lower brain stem . The largest known cells are unfertilised ostrich egg cells, which weigh 3.3 pounds.
The picture above is what an animal cell looks like.
In this chapter we learnt about the different type of cells, tissues and organs.
The cell is the functional basic unit of life. It was discovered by Robert Hooke and is the functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. Some organisms, such as most bacteria, are unicellular (consist of a single cell). Other organisms, such as humans, are multicellular. Humans have about 100 trillion or 1014 cells; a typical cell size is 10 µm and a typical cell mass is 1 nanogram. The longest human cells are about 135 µm in the anterior horn in the spinal cord while granule cells in the cerebellum, the smallest, can be some 4 µm and the longest cell can reach from the toe to the lower brain stem . The largest known cells are unfertilised ostrich egg cells, which weigh 3.3 pounds.
In 1835, before the final cell theory was developed, Jan Evangelista Purkyně observed small "granules" while looking at the plant tissue through a microscope. The cell theory, first developed in 1839 by Matthias Jakob Schleiden and Theodor Schwann, states that all organisms are composed of one or more cells, that all cells come from preexisting cells, that vital functions of an organism occur within cells, and that all cells contain the hereditary information necessary for regulating cell functions and for transmitting information to the next generation of cells.
The word cell comes from the Latin cellula, meaning "a small room". The descriptive term for the smallest living biological structure was coined by Robert Hooke in a book he published in 1665 when he compared the cork cells he saw through his microscope to the small rooms monks lived in.
There are different cell parts with different functions. The cell parts below are those found in
animal cells.
Nucleus: Contains genetic material. Directs activities of the cell. Controls cell division and
growth.
growth.
Cytoplasm: Fills the cell and contains substances needed for reactions in the cell.
Mitochondrion: Cellular respiration.
Cell membrane: It is selectively pemeable to control movement of substances into and out of the
cell.
cell.
Vacuole: Storage compartment for water, sugars and pigments.
Ribosome: Produces protein from a set of genetic instructions.
Cell wall: Provides support for plant cell.
However, animal cells do not have chloroplasts nor do they have cell walls.
Ribosome: Produces protein from a set of genetic instructions.
Cell wall: Provides support for plant cell.
However, animal cells do not have chloroplasts nor do they have cell walls.
The two types of cells are animal cells and plant cells. Animal cells are different from plant cells in their
structure.
structure.
This is what a plant cell looks like.
See the difference?
Yes, a plant cell has cell wall and chloroplasts while the animal cell doesn't. Also, the plant cell's vacuole is
much larger than an animal cell's and there is only one vacuole in a plant cell.
Now, tissues.
Tissues are really cells grouped together. There are a few kinds of tissues which perform different functions.
Plants and animals' tissues are different too. Plants have Epidermal, Xylem, Phloem, Photosynthetic and palisade tissues.
Animal tissues include epithelial, muscle, connective, nerve and glandular tissues.
All these tissues form organs.
Examples of plant organs are roots, stems, leaves. While examples of animal organs are the stomach, liver and
small intestines.
These form systems such as respiratory system & digestive system.
Plants and animals' tissues are different too. Plants have Epidermal, Xylem, Phloem, Photosynthetic and palisade tissues.
Animal tissues include epithelial, muscle, connective, nerve and glandular tissues.
All these tissues form organs.
Examples of plant organs are roots, stems, leaves. While examples of animal organs are the stomach, liver and
small intestines.
These form systems such as respiratory system & digestive system.
Wednesday, September 7, 2011
Elements, Mixtures and Compounds
This topic covers elements, mixtures and compounds.
Elements: Elements are substances which cannot be split into simpler substances by any known chemical methods. They are all given a name and a chemical symbol. Altogether there are 109 known elements, and are all listed in the periodic table with their atomic mass, chemical symbol and atomic number.
The table below is the periodic table.
Mixtures: Mixtures are elements mixed together and they can be separated by physical methods such as distillation, filtration, using a magnet and evaporation. Mixtures also have properties similar to its constituent elements. Mixtures such as air are combinations of nitrogen, carbon dioxide, oxygen, etc.etc.
Compounds: Compounds are elements( 2 or more) chemically combined. They can only be separated by chemical methods such as burning, electrolysis etc.etc. Compounds have properties different from its constituent elements. Examples of such compounds are water, magnesium oxide and sodium chloride. However, compounds can only form if the elements are joined in the correct proportion. Eg. Water only forms when 1 part hydrogen mixes with 1 part oxygen.
Elements: Elements are substances which cannot be split into simpler substances by any known chemical methods. They are all given a name and a chemical symbol. Altogether there are 109 known elements, and are all listed in the periodic table with their atomic mass, chemical symbol and atomic number.
The table below is the periodic table.
Mixtures: Mixtures are elements mixed together and they can be separated by physical methods such as distillation, filtration, using a magnet and evaporation. Mixtures also have properties similar to its constituent elements. Mixtures such as air are combinations of nitrogen, carbon dioxide, oxygen, etc.etc.
Compounds: Compounds are elements( 2 or more) chemically combined. They can only be separated by chemical methods such as burning, electrolysis etc.etc. Compounds have properties different from its constituent elements. Examples of such compounds are water, magnesium oxide and sodium chloride. However, compounds can only form if the elements are joined in the correct proportion. Eg. Water only forms when 1 part hydrogen mixes with 1 part oxygen.
Sunday, September 4, 2011
Kinetic Particle Theory( Brownian Motion)
The Brownian Motion-
Brownian motion (named after the botanist Robert Brown) or pedesis is the presumably random drifting of particles suspended in a fluid (a liquid or a gas) or the mathematical model used to describe such random movements, which is often called a particle theory.
The mathematical model of Brownian motion has several real-world applications. An often quoted example is stock market fluctuations, however, movements in share prices may arise due to unforeseen events which do not repeat themselves.
Brownian motion is among the simplest of the continuous-time stochastic (or probabilistic) processes, and it is a limit of both simpler and more complicated stochastic processes. This universality is closely related to the universality of the normal distribution. In both cases, it is often mathematical convenience rather than the accuracy of the models that motivates their use. This is because Brownian motion, whose time derivative is everywhere infinite, is an idealised approximation to actual random physical processes, which always have a finite time scale.
Brownian motion is not to be mistaken with kinetic particle theory, which I had thought were the same. However, Brownian motion is a form of kinetic particle theory,
Above is a video of Brownian motion.
Brownian motion was first observed by Robert Brown and was a botanist.
My reflections for this topic:
This topic was rather easy at first, but it became harder later as there were more things to remember. Also, Brownian motion is just a form of kinetic particle theory so it is only a small part of kinetic particle theory.
Brownian motion was first observed by Robert Brown and was a botanist.
My reflections for this topic:
This topic was rather easy at first, but it became harder later as there were more things to remember. Also, Brownian motion is just a form of kinetic particle theory so it is only a small part of kinetic particle theory.
Saturday, September 3, 2011
SI units and Quantities and Prefixes
Physical Quantities, SI Units
A physical quantity is a quantity which can be measured.
Examples of some of them are length, volume, mass, time, temperature, etc.
Examples of some of them are length, volume, mass, time, temperature, etc.
A non-physical quantity is one which cannot be measured.
Examples of some of them are beauty, kindness, humour, sadness, untidiness, etc.
Examples of some of them are beauty, kindness, humour, sadness, untidiness, etc.
Since 1960, scientists from different parts of the world have agreed to adopt a single system of units called the SI Units (SI stands for Système International d’Unités in French). This system is an adaptation of the metric system.
There are altogether seven basic quantities: length, mass, time, electric current, thermodynamic temperature, luminous intensity and amount of substance.
The International System of Units (SI units)
The table below is the SI units
| Name | Unit symbol | Quantity | Typical Symbol for Variables |
|---|---|---|---|
| metre | m | length | l (a lowercase L) |
| kilogram | kg | mass | m |
| second | s | time | t |
| ampere | A | electric current | I (a capital i) |
| kelvin | K | thermodynamic temperature | T |
| candela | cd | luminous intensity | Iv (a capital i with lowercase non-italicized v subscript) |
| mole | mol | amount of substance | n |
The SI unit is made to let foreign scientists at international events to understand the units as their native units are different from other countries'.
Prefixes:
Micro:-Symbol: m, Value-10 to the power of negative 6, also 1 millionth, or 0.0000001
Milli:
-Symbol: m Value: 10 to the power of negative 3, also 1 thousandth, or 0.001
Centi:
-Symbol: c Value: 10 to the power of negative 2, also 1 hundredth, or 0.01
Deci:
-Symbol: d Value: 10 to the power of negative 1, also 1 tenth, or 0.1
Kilo:
-Symbol: k Value : 10 to the power of 3, also 1 thousand, or 1000
Mega
-Symbol: M Value: 10 to the power of 6, also 1 million, or 100000
This topic is quite easy, as I only need to memorize the prefixes and SI units. I thought of a technique to remember the symbol Milli and Mega, as their symbols are m & M and hence can be quite confusing. for Mega, I told myself capital m-ega, so when I combined them, it becomes Captaimega. When I knew one, i knew the other m was milli, so this method was effective for me.
This topic is quite easy, as I only need to memorize the prefixes and SI units. I thought of a technique to remember the symbol Milli and Mega, as their symbols are m & M and hence can be quite confusing. for Mega, I told myself capital m-ega, so when I combined them, it becomes Captaimega. When I knew one, i knew the other m was milli, so this method was effective for me.
Thursday, September 1, 2011
Measurements
In this chapter, I learnt about measurements- Vernier calipers, Micrometer screw gauge and how to avoid parallex error and zero error.
Measurements: There are many items used to measure objects, and the type of measurements from thickness to weight, length to speed. I learnt how to use the Vernier caliper and micrometer screw gauge in this chapter.Vernier caliper: It is used to measure things accurately to 0.01cm, and can also be used to measure the diameter of spheres. This is what it looks like-
1. Preparation to take the measurement, loosen the locking screw and move the slider to check if the vernier scale works properly. Before measuring, do make sure the caliper reads 0 when fully closed. If the reading is not 0, adjust the caliper’s jaws until you get a 0 reading. If you can’t adjust the caliper, you will have to remember to add to subtract the correct offset from your final reading. Clean the measuring surfaces of both vernier caliper and the object, then you can take the measurement.
2. Close the jaws lightly on the item which you want to measure. If you are measuring something round, be sure the axis of the part is perpendicular to the caliper. Namely, make sure you are measuring the full diameter. An ordinary caliper has jaws you can place around an object, and on the other side jaws made to fit inside an object. These secondary jaws are for measuring the inside diameter of an object. Also, a stiff bar extends from the caliper as you open it that can be used to measure depth.
3. How to read the measured value:
1), Read the centimeter mark on the fixed scale to the left of the 0-mark on the vernier scale. (10mm on the fixed caliper)
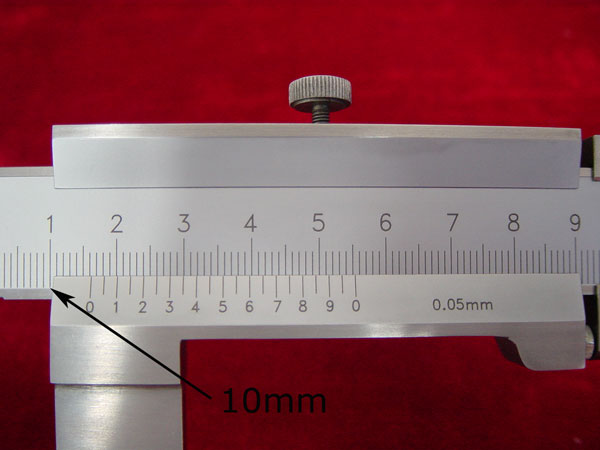
2). Find the millimeter mark on the fixed scale that is just to the left of the 0-mark on the vernier scale. (6mm on the fixed caliper)
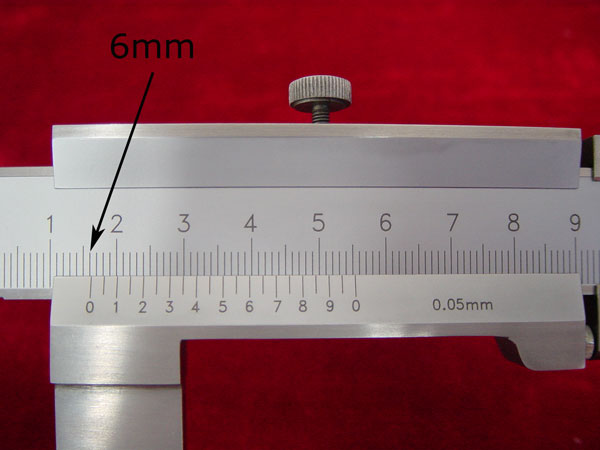
3). Look along the ten marks on the vernier scale and the millimeter marks on the adjacent fixed scale, until you find the two that most nearly line up. (0.25mm on the vernier scale)
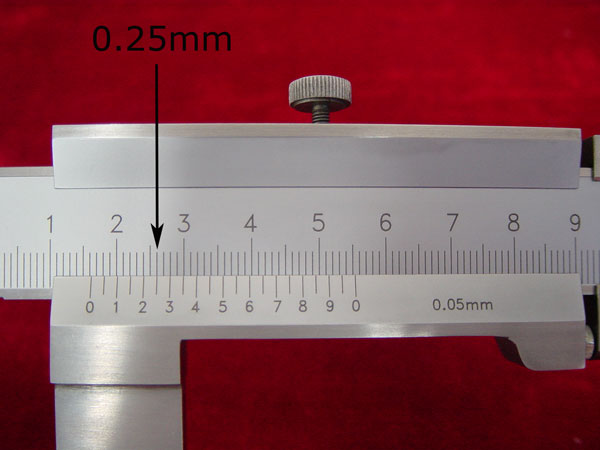
4). To get the correct reading, simply add this found digit to your previous reading. (10mm + 6mm + 0.25mm= 16.25 mm)
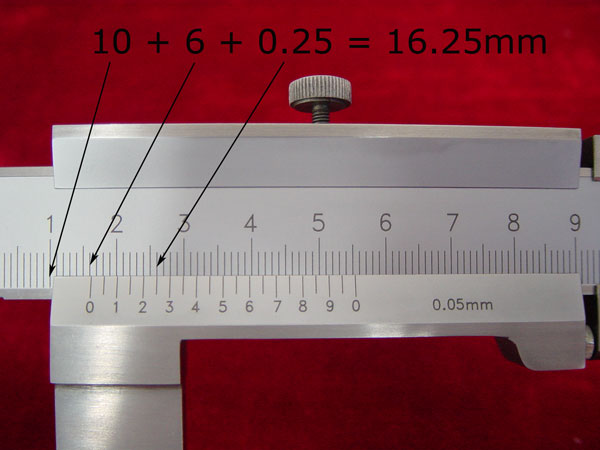
4.Maintenance
Clean the surface of the vernier caliper with dry and clean cloth (or soaked with cleaning oil) and stock in a dry environment if it stands idle for a long time.
Micrometer Screw Gauge: The micrometer screw gauge is used for measuring the diameter of fine wires, the thickness of paper and similar small distances, a micrometer screw gauge (commonly shortened to micrometer) is used. It consists of a frame, an anvil, a thimble, a ratchet stop, screw, a spindle, a barrel and a lock nut. The method of operation is as follows-
Screw-gauge
The screw has a known pitch such as 0.5 mm. Pitch of the screw is the distance moved by the spindle per revolution. Hence in this case, for one revolution of the screw the spindle moves forward or backward 0.5 mm. This movement of the spindle is shown on an engraved linear millimeter scale on the sleeve. On the thimble there is a circular scale which is divided into 50 or 100 equal parts.
When the anvil and spindle end are brought in contact, the edge of the circular scale should be at the zero of the sleeve (linear scale) and the zero of the circular scale should be opposite to the datum line of the sleeve.
Zero error- Zero error occurs when your instrument has an incorrect reading when measuring something that should read zero. E.g- Your weighing scale has nothing on it, but it doesn't read zero. Instead, the scale reads a different mass although there is nothing on it.
Measurements: There are many items used to measure objects, and the type of measurements from thickness to weight, length to speed. I learnt how to use the Vernier caliper and micrometer screw gauge in this chapter.Vernier caliper: It is used to measure things accurately to 0.01cm, and can also be used to measure the diameter of spheres. This is what it looks like-
The basic steps are as follows:
1. Preparation to take the measurement, loosen the locking screw and move the slider to check if the vernier scale works properly. Before measuring, do make sure the caliper reads 0 when fully closed. If the reading is not 0, adjust the caliper’s jaws until you get a 0 reading. If you can’t adjust the caliper, you will have to remember to add to subtract the correct offset from your final reading. Clean the measuring surfaces of both vernier caliper and the object, then you can take the measurement.
2. Close the jaws lightly on the item which you want to measure. If you are measuring something round, be sure the axis of the part is perpendicular to the caliper. Namely, make sure you are measuring the full diameter. An ordinary caliper has jaws you can place around an object, and on the other side jaws made to fit inside an object. These secondary jaws are for measuring the inside diameter of an object. Also, a stiff bar extends from the caliper as you open it that can be used to measure depth.
3. How to read the measured value:
1), Read the centimeter mark on the fixed scale to the left of the 0-mark on the vernier scale. (10mm on the fixed caliper)

2). Find the millimeter mark on the fixed scale that is just to the left of the 0-mark on the vernier scale. (6mm on the fixed caliper)

3). Look along the ten marks on the vernier scale and the millimeter marks on the adjacent fixed scale, until you find the two that most nearly line up. (0.25mm on the vernier scale)

4). To get the correct reading, simply add this found digit to your previous reading. (10mm + 6mm + 0.25mm= 16.25 mm)

4.Maintenance
Clean the surface of the vernier caliper with dry and clean cloth (or soaked with cleaning oil) and stock in a dry environment if it stands idle for a long time.
Micrometer Screw Gauge: The micrometer screw gauge is used for measuring the diameter of fine wires, the thickness of paper and similar small distances, a micrometer screw gauge (commonly shortened to micrometer) is used. It consists of a frame, an anvil, a thimble, a ratchet stop, screw, a spindle, a barrel and a lock nut. The method of operation is as follows-
The screw has a known pitch such as 0.5 mm. Pitch of the screw is the distance moved by the spindle per revolution. Hence in this case, for one revolution of the screw the spindle moves forward or backward 0.5 mm. This movement of the spindle is shown on an engraved linear millimeter scale on the sleeve. On the thimble there is a circular scale which is divided into 50 or 100 equal parts.
When the anvil and spindle end are brought in contact, the edge of the circular scale should be at the zero of the sleeve (linear scale) and the zero of the circular scale should be opposite to the datum line of the sleeve.
Zero error- Zero error occurs when your instrument has an incorrect reading when measuring something that should read zero. E.g- Your weighing scale has nothing on it, but it doesn't read zero. Instead, the scale reads a different mass although there is nothing on it.
Zero Error for Vernier caliper-
Positive zero error is when the jaws are closed and the zero on the vernier scale is to the right of the zero of the main scale.The measurement taken with this pair of vernier calipers will be MORE than the actual result. To get the correct value, the zero error must be subtracted from each reading. Eg: The zero error (0.03) is subtracted from the reading. The actual reading is thus (eg) 2.35-0.03cm= 2.32
Negative error is when the jaws and closed and the zero of the vernier scale is to the left or the zero of the main scale. The measurement taken with this pair if vernier calipers will be LESS than the actual value. To get the correct value, the numerical value of the zero error must be ADDED to each reading. Eg: The 4th line on the vernier scale is in line with the marking on the main scale. The zero error (0.10-0.04cm=0.06cm) is added to the rading. The actual reading is thus 2.35+0.06cm=2.41cm.
Negative error is when the jaws and closed and the zero of the vernier scale is to the left or the zero of the main scale. The measurement taken with this pair if vernier calipers will be LESS than the actual value. To get the correct value, the numerical value of the zero error must be ADDED to each reading. Eg: The 4th line on the vernier scale is in line with the marking on the main scale. The zero error (0.10-0.04cm=0.06cm) is added to the rading. The actual reading is thus 2.35+0.06cm=2.41cm.
Zero error for micrometer screw gauge-
When the anvil and spindle end are brought in contact, the edge of the circular scale should be at the zero of the sleeve (linear scale) and the zero of the circular scale should be opposite to the datum line of the sleeve. If the zero is not coinciding with the datum line, there will be a positive or negative zero error as shown in figure below.
Zero error in case of screw gaugeWhile taking a reading, the thimble is turned until the wire is held firmly between the anvil and the spindle.
The least count of the micrometer screw can be calculated using the formula given below:
Least count

= 0.01 mm
Learning to read the vernier caliper and micrometer screw gauge is not easy for me, and I always failed to understand how to read them and why I needed to read them as such. Hence, I failed my first pop quiz on Vernier caliper and micrometer screw gauge. However, with help from my teacher and friends, I gradually grasped the concept on how to use these instruments and how to correct zero error. These instruments may prove hard to learn at first, but if you persist you will eventually learn.
The least count of the micrometer screw can be calculated using the formula given below:
Least count


= 0.01 mm
Learning to read the vernier caliper and micrometer screw gauge is not easy for me, and I always failed to understand how to read them and why I needed to read them as such. Hence, I failed my first pop quiz on Vernier caliper and micrometer screw gauge. However, with help from my teacher and friends, I gradually grasped the concept on how to use these instruments and how to correct zero error. These instruments may prove hard to learn at first, but if you persist you will eventually learn.
Science as an Inquiry
This topic teaches me the attitudes of good scientists, variables, safety rules in the laboratory, hazard symbols, lab equipments, Bunsen burner's 2 types of flames.
Attitudes of good scientists: I learnt that good scientists should be curious, persevere till the end, be optimistic- don't let failure stop you from achieving your goals. Good scientists should also be open-minded: be open to others' suggestions and criticisms. They want to help you achieve your aims, so don't take their words too harshly and personally. Instead, reflect upon your own mistakes and make an effort to strive better.
The safety rules when in a laboratory: - Do not tamper with the electrical mains and other fittings in the laboratory.- Do not eat, drink or play in the laboratory.- Do not taste any chemicals unless otherwise instructed by the teacher.- Do not pour any unused chemicals back into its container to avoid contamination.- Handle all apparatus and chemicals carefully and correctly. Always check the label on the container before using the substance it contains.- Wash your hands after all laboratory work.- Do not remove any apparatus or chemicals from the laboratory.- Return the apparatus to their proper storage places after cleaning.- Work tidily. Wash up all used apparatus and dispose of the waste correctly. These are the guidelines that are to be followed at all times. I have also had an experience of accidentally smashing a test tube before, but luckily I smashed it in the sink, so it was not that much of a hazard. However, I must still be careful in a laboratory as I will be dealing with more dangerous items like concentrated acid.
Hazard symbols: There are many hazard symbols which are quite easy to recognize and they are used quite commonly in our lives. There is the flammable sign, explosive,corrosive,poison, irritating or stimulating and radioactive signs. These signs help us to recognize unknown substances and how to handle them.
Bunsen burner- The Bunsen burner was invented by scientist Robert Bunsen and his partner Peter Desaga in 1854. The aim was to create a sootless, non-luminous flame by mixing the gas with air in a controlled fashion before combustion. Now, this design is used in laboratories all over the world. A Bunsen burner has two main types of flames. A luminous and non-luminous flame. A luminous flame - Occurs when the air-holes are closed insufficient air is allowed to mix with the gas therefore gas does not burn completely.
Attitudes of good scientists: I learnt that good scientists should be curious, persevere till the end, be optimistic- don't let failure stop you from achieving your goals. Good scientists should also be open-minded: be open to others' suggestions and criticisms. They want to help you achieve your aims, so don't take their words too harshly and personally. Instead, reflect upon your own mistakes and make an effort to strive better.
The safety rules when in a laboratory: - Do not tamper with the electrical mains and other fittings in the laboratory.- Do not eat, drink or play in the laboratory.- Do not taste any chemicals unless otherwise instructed by the teacher.- Do not pour any unused chemicals back into its container to avoid contamination.- Handle all apparatus and chemicals carefully and correctly. Always check the label on the container before using the substance it contains.- Wash your hands after all laboratory work.- Do not remove any apparatus or chemicals from the laboratory.- Return the apparatus to their proper storage places after cleaning.- Work tidily. Wash up all used apparatus and dispose of the waste correctly. These are the guidelines that are to be followed at all times. I have also had an experience of accidentally smashing a test tube before, but luckily I smashed it in the sink, so it was not that much of a hazard. However, I must still be careful in a laboratory as I will be dealing with more dangerous items like concentrated acid.
Hazard symbols: There are many hazard symbols which are quite easy to recognize and they are used quite commonly in our lives. There is the flammable sign, explosive,corrosive,poison, irritating or stimulating and radioactive signs. These signs help us to recognize unknown substances and how to handle them.
Bunsen burner- The Bunsen burner was invented by scientist Robert Bunsen and his partner Peter Desaga in 1854. The aim was to create a sootless, non-luminous flame by mixing the gas with air in a controlled fashion before combustion. Now, this design is used in laboratories all over the world. A Bunsen burner has two main types of flames. A luminous and non-luminous flame. A luminous flame - Occurs when the air-holes are closed insufficient air is allowed to mix with the gas therefore gas does not burn completely.
- Carbon particles are produced.
- Orange in colour.
- Appear flickering and unsteady.
- Not very hot.
A non-luminous flame- - Occurs when the air-holes are opened, allowing sufficient air into the burner therefore gas is burn completely.
- Blue in colour.
- Burns steadily.
- Hotter than Luminous flame.
- Hottest part of the flame is just above
the tip of the dark blue zone.
the tip of the dark blue zone.
So these are the two types of flames that are normally used. When boiling water, most of us use the non-luminous flame as it is hotter than the luminous flame and it boils the water faster. The former is hence preferred over the latter.
Variables- There are 3 types of variables in science: independent variables, dependent variables and constant variables.
1:Independent variables-
An independent variable is a one whose values are chosen by the experimenter. In other words, the values of these variables are independent of the participant's behaviour.
For the independent variable to be manipulated, at least two levels must be present in an experiment. The most basic way to manipulate is by creating one experimental group (the group receiving the treatment) and a control group (exactly like the experimental group, except without the experimental treatment). The performances of the two groups in regard to the dependent measure are then compared to assess the effect of the independent variable.
2:Dependent variable-
The variable whose value you observe and record in experimental designs is the called the dependent variable (or dependent measure). In other words, the dependent variable value depends on the behaviour of the participant, rather than being set by the experimenter.
3: Constant variable-
Those factors in the experiment that the scientist attempts to keep constant.
The control is the experiment with the absence of the independent variable.
So, these are the things I have learnt in 'Science as an Inquiry'. Thanks for reading!
Tuesday, August 30, 2011
Welcome to my Science e-Portfolio
Hi!!!! Welcome to my Science e-Portfolio!!!! This website will take you through my journey of this year's Lower Secondary Science lessons and my experiences. Enjoy!
Subscribe to:
Posts (Atom)











